Due to frequent observed side effects in vaccinated young people, the constant vaccination commission (STIKO) is now changing their recommendation: children from 12 and adolescents and adults under the age of 30 should only be vaccinated with the corona vaccine from Biontech and no longer with that of Moderna.The two shares are already reacting in pre-exchangeable US trading and Germany's stock exchanges.
According to STIKO, current registration analyzes showed that heart muscle and pericarditis were observed more frequently after moderna vaccination (Spikevax) than after the Biontech vaccination (Comirnaty).The new recommendation now applies to both the basic immunization and possible refreshing vaccinations, according to the STIKO.Even if another vaccine had been used beforehand, Biontech should now be used for further vaccinations, it was said.
The expert committee refers to safety data from the Paul-Ehrlich Institute (PEI) and international data.The STIKO also advises that pregnant women should also get independent of age Biontech, even if there are no comparative safety data for the two vaccines for them.
It is not yet a final stiko recommendation.The draft decision for the corresponding update of the COVID-19 vaccination recommendation was to vote on specialist groups and countries, it said.Changes are therefore still possible.
Side effects "mostly mild"
The course of the cardiac muscle and pericardium infections is "mostly mild" according to the previously available safety reports, explained the STIKO.As the PEI indicates in its security report, the inflammation occurred particularly after the second vaccination.The first complaints would typically be noticed within a few days after the piks.The STIKO emphasized that for humans from 30 there was no increased risk of the two inflammation after moderna vaccination.
The vaccines of Biontech/Pfizer and the US manufacturer Moderna are similar in some points: both are MRNA vaccines, two doses are administered for basic immunization.Since the introduction of both preparations, it has been known that "in rare cases" cardiac muscle and/or pericarditis can result in younger people, writes the stiko.
The chairman Thomas Mertens said the dpa on Wednesday that the hypothesis was that the more frequently recorded cases in the Moderna vaccine could be related to its comparatively higher mRNA dosage.
France's highest health authority also advises people under 30 for the same reasons from Moderna as it emerged from a recommendation from the authority on Monday evening.
Stock exchanges.Briefing NewsletterBleiben Sie über die neuesten Entwicklungen bei spannenden Unternehmen und an der Börse auf dem Laufenden. Lesen Sie das Stock exchanges.Briefing.- the daily newsletter of the shareholder.For free.
Moderna requested from EMA approval for children
Moderna had announced the previous day that the European Pharmaceutical Agency also applied for vaccination approval for children aged six to eleven (the shareholder reported).There is still no approved vaccine for children under the age of 12 in Europe.Modernas Corona vaccine is approved in the United States from the age of 18, in the EU for children and adolescents from the age of 12.In the United States, the Corona vaccine from Biontech /Pfizer for children between the ages of five and eleven had already received emergency approval
For Moderna, the STIKO Council is not good news for younger people on Biontech.But when looking at the numbers, the picture is relativized.Of the total of 447 million EU citizens, a good 60 million belong to the relevant group of 12 to U30 year old.Many of them are already vaccinated.In addition, new potential vaccine candidates are waiting for moderna injections in the U12 children's group.
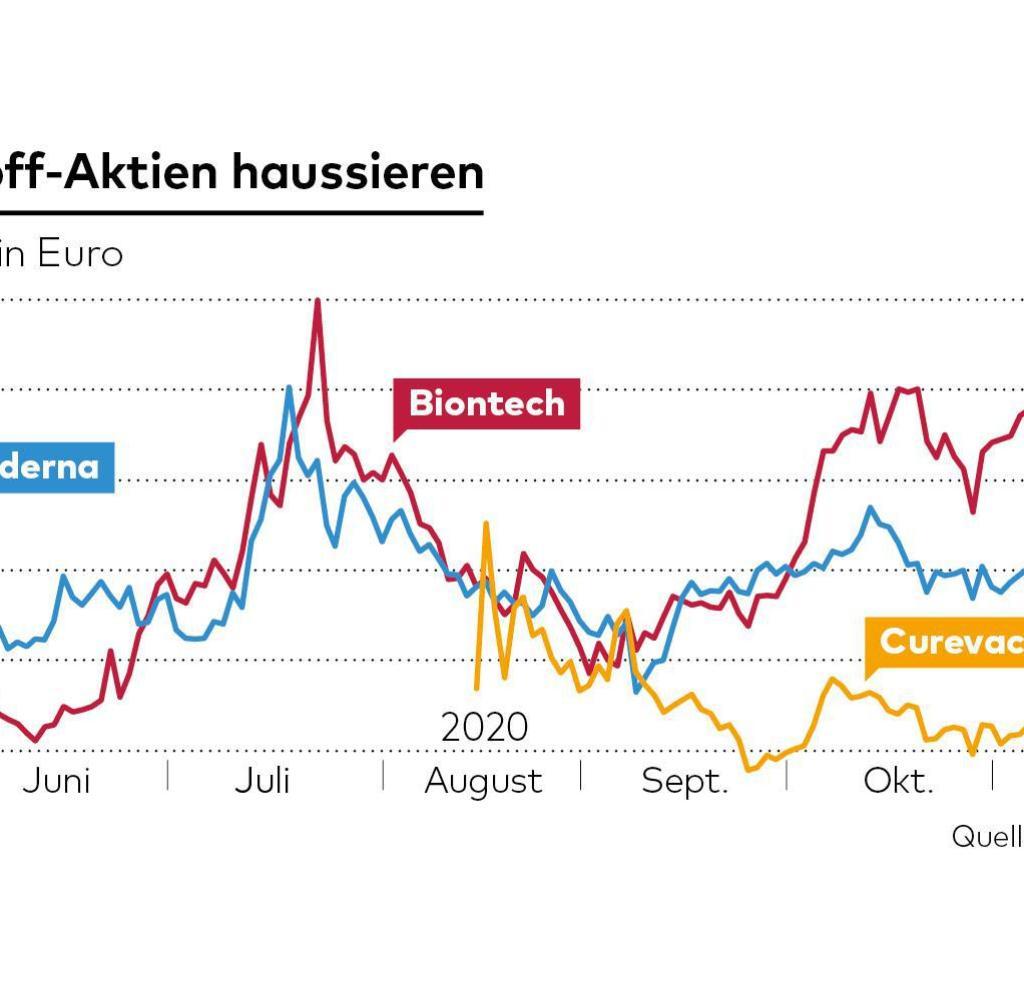
On the stock exchange, both vaccine manufacturers were recently significantly under pressure.On Wednesday, the Moderna share is a little weaker than the Biontech share.The two vaccine manufacturers have been largely parallel since the beginning of the year-with an advantage for Biontech (see course development via tradegate).
Moderna(WKN: A2N9D9)Both pharmaceutical groups have corona potential in the long term in view of the attractive new infections and upcoming booster vaccinations.Both also have other products in the pipeline.The development of mRNA-based technology opens new doors to both companies.
Despite all the current course turbulence, the shareholder remains confident for the further development of both pharmaceutical companies.Biontech and Moderna are ongoing recommendations.Biontech may be ahead with a view to promising oncology projects.
If you do not want to rely on individual values among the vaccine shares, take a look at the shareholder's vaccine stock index.The correction since September has pressed the index with its eight values to an interesting level of entry.
You can find more about the vaccine stock index here.
(With material from dpa-AfX)
Reference to conflicts of interest:
Der Vorstandsvorsitzende und Mehrheitsinhaber der Herausgeberin Stock exchangesmedien AG, Herr Bernd Förtsch, ist unmittelbar und mittelbar Positionen über die in der Publikation angesprochenen nachfolgenden Finanzinstrumente oder hierauf bezogene Derivate eingegangen, die von der durch die Publikation etwaig resultierenden Kursentwicklung profitieren können: BioNTech.
Note on conflicts of interest according to § 85 WPHG: Shares of Biontech are in the shareholder depot and in the lever depot.









How to get the perfect look for Cos...
Test winner at Stiftung Warentest:...
Dry elbows: This is how brittle ski...
Cream for Rosacea: The Best Creams